Researchers In Israel Are Using Solaris Bioreactors to Develop Vaccine Against Novel Coronavirus COVID-19
Over the last 4 years, Galilee Research Institute MIGAL has developed the vaccine for another type of Coronavirus that occurs in poultry (Infectious Bronchitis Virus/IBV); and believes that the genetic similarity between IBV and COVID-19 will simplify human vaccine synthesis. MIGAL researchers claimed in early March 2020 to be able to develop an oral vaccine for testing in 3 months due to the similar DNA structure with the current IBV vaccine. To simplify production, MIGAL turned to Solaris to provide its most modern Jupiter bioreactors.

Solaris Race to find the vaccine for COVID-19:
Original Italian Version | Translated to English
Israel increasingly close to a vaccine for Covid-19: the Solaris pelmet in the team
Original Italian Version
Article about MIGAL:
Israeli researchers near Covid-19 vaccine development
"Solaris Biotech Solutions is proud to make our experience and cutting-edge devices available to meet this global challenge" said Solaris CEO Matteo Brognoli. "We are experts in the medical and pharmaceutical fields, and we are sure we can help the researches get great results with our bioreactors."
What is the Coronavirus vaccine and how will Solaris Bioreactors make it?
Vaccine Definitions
Vaccines are paramount tools in public health that have saved billions of lives. They use the specific markers in infectious diseases that our immune system can detect and develop resistance against. Before explaining how the COVID-19 vaccine is made, two terms need defining.
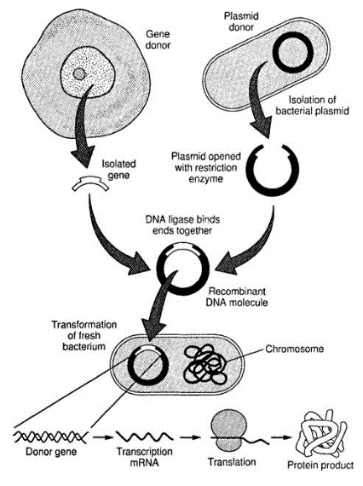
Recombinant DNA technology: the combination and study of DNA from different species
The central dogma of biology dictates that coding DNA transcribes to RNA which translates into proteins. No matter which species the coding DNA exists in, it has the potential to transcribe and translate into proteins (reference: ebook).
For example: To produce human insulin for diabetics, David Goeddel et al. inserted the human insulin gene into E. coli bacteria in 1978. By 1982 recombinant human insulin hit the market. This technology enabled faster and cheaper production for diabetic patients all around the world and removed the need to source insulin from animal organs (history of insulin).
Viral Vectors: Non-harmful viruses that deliver safe, recombinant DNA of harmful diseases for our immune system to attack
By putting non-infectious DNA from harmful diseases into a non-infectious virus (called a viral vector): scientists are working on making vaccines and treating diseases such as Ebola and multiple types of cancers (Vector vaccine review | Ebola vector vaccine | Therapeutic cancer vaccines). When viral vectors deliver recombinant DNA into our cells, our cells turn that DNA into specific, immune system detectable, noninfectious proteins called antigens that are unique to the pathogen. In turn, our immune system detects and neutralizes the antigen while at the same time generating resistance and memory in case of future contact with the pathogen.
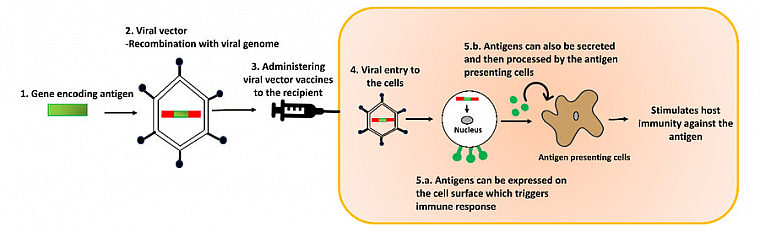
What are bioreactors, how do they make vaccines, and why Solaris?
In the most basic sense, bioreactors are big, sterile vessels that control conditions for cells to grow in: such conditions as temperature, acidity (pH), or dissolved oxygen (DO). By using recombinant DNA technology (as well as many other methods), researchers can induce cells into making products that we need, such as vaccines, drugs, foods, plastics, and fuels.
Depending on the types of cells needed for production, scientists need the customization and flexibility of controlling whichever parameters they need. This is why Galilee Research Institute MIGAL is using Solaris bioreactors to make a viral vector COVID-19 vaccine using recombinant DNA technology.
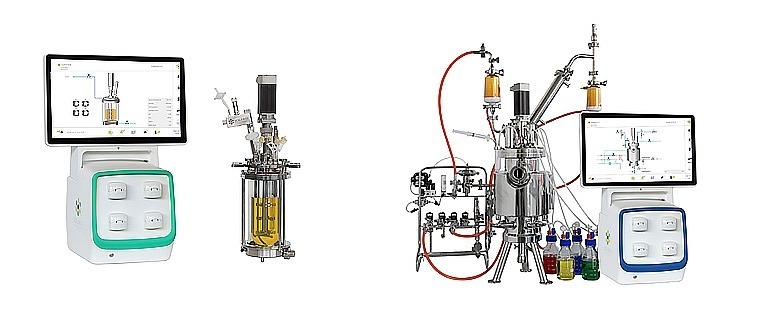
To make a product or protein of interest, one must sterilize and control the environment within the bioreactor, add media, cells, and maybe an inducer chemical to activate production. Galilee Research institute MIGAL have cells making viral vectors with Solaris bioreactors and they trust our technology to scale for growth.
Benefits of Solaris Bioreactors
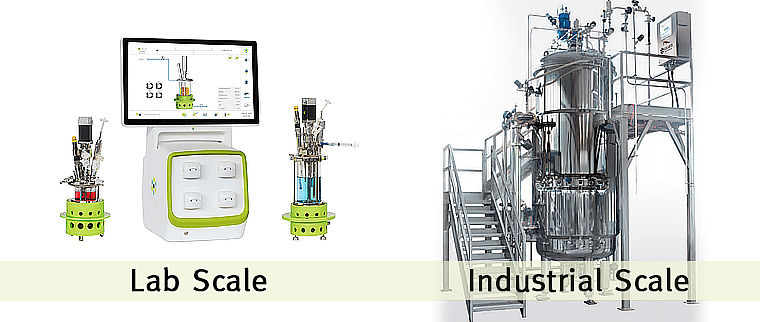
- Customizable and scalable products ranging from 200mL to 30,000L in size.
- Intuitive, easy to use software that can control up to 24 bioreactors at once from one touch screen (no programming required.)
- CGMP & FDA 21 CFR Part 11 compliant.
- Competitive pricing.
- Short lead times.
- Personable, rapid, and responsive service.
From laboratory research to pilot plant to industrial scale, Solaris is the reliable solution for scaling up.
Who else trusts Solaris?
With the customizability, scalability, fine control, and ease of use that Solaris bioreactors offer; universities, companies, research institutions, and startups from all around the world have come to expect the best from this growing company in Northern Italy.
Please, don't hesitate to email or call us about our expertise and expanding responsibility in bioprocesses around the world. Solaris wants to hear from you!