The Challenges of Scaling Up and Selecting the Right Pilot Scale Fermenter
Pilot scale production is “make or break” for most bioprocesses and is often the limiting step for bringing a product to market. Moving from the benchtop and R&D scale to a pilot plant is the safest way to establish what aspects of production and process parameters need to be considered for manufacturing.
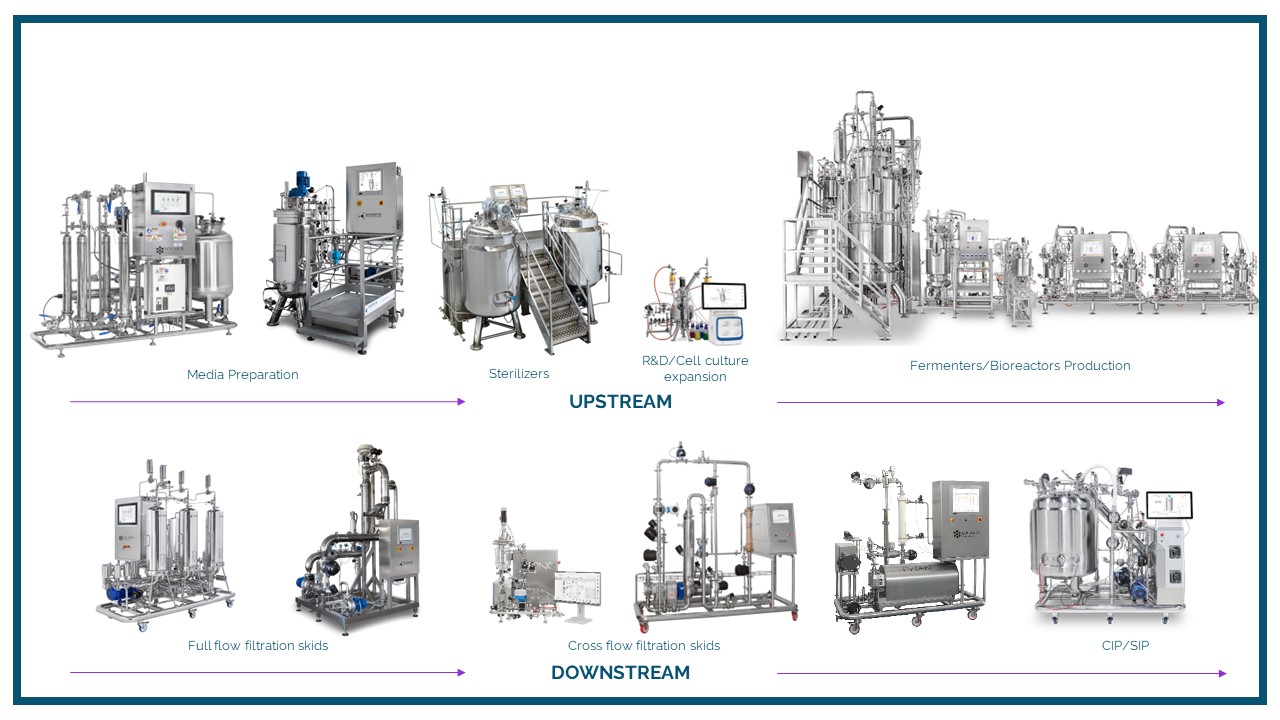
The fermentation is often the hardest part of scaling up. Pilot fermentations require an intimate knowledge of not just the biology of the organism, but the equipment required to make the process successful. It is essential to understand the physical and chemical limitations of your system and how to address the challenges of scaling up. Selecting the correct bioreactor for your process is key to achieving success and optimizing product yield, quality, and stability.
Reasons for Pilot Plant Production
The purpose of a pilot/demo plant is to translate a laboratory process into a small-scale industrial process. Building a pilot facility is one of the most expensive parts of production development, so it is crucial that this phase is completed on time and on budget. For this reason, pilot manufacturing is often outsourced, leading to issues with compatible facilities/equipment, process control, and contamination.
Whichever route is taken, the most important role of a pilot plant is to demonstrate proof of concept for a process. A pilot plant will provide material for downstream processing and quality assessment. It is also necessary to optimize the process at scale, often tweaking lessons learned at the benchtop to translate into full scale manufacturing.
Ultimately the goal of the pilot plant is to develop and optimize the process for production manufacturing.
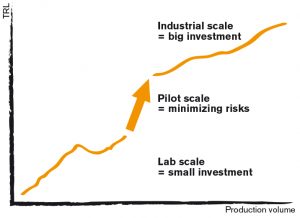
Key Considerations for Pilot Plant Planning
To build a successful pilot plant, it is important to know what your goals are from the start. Understanding the production targets and downstream processing allow for assessment of risk and help to develop contingencies to mitigate issues that may arise.
A successful pilot plant will demonstrate a predictable, cost effective, and robust process that produces product that meets quality specifications. The process needs to meet the regulatory requirements set for that product/industry. Often this means that the process must meet Good Manufacturing Practices (GMP) and be CFR 211 compliant. Establishing the blueprint for process validation at the pilot scale can ease the transition into full scale production.
Pilot Plant Mantra: “Get it right, and get it right the first time”
As with any large project, the most important factor is often the budget available. It is important to perform economic analysis to estimate capital costs (CAPEX) as well as the total manufacturing costs (OPEX). Pilot plants are an excellent way to trial production, but also understand the costs associated with manufacturing.

The expense of running a pilot process is often prohibitory, with fermentation iterations being the biggest cost. Maximizing development speed and process fidelity are paramount. A return on investment must be demonstrated in a defined time-frame or a project is doomed to failure.

Challenges for Scaling Up Bioprocesses
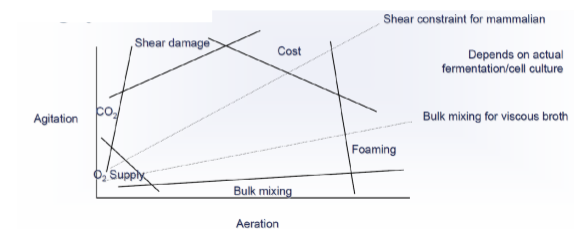
The challenges for pilot scale process optimization are often different than those for lab/R&D scale. Many of the same parameters are considered (kLa/OTR, heat transfer, shear, etc…) but the inherent dynamics of a system change when the volume is increased. Additionally, the equipment and controls available for industrial processes are fundamentally different from benchtop bioreactors. Thus, for a variety of reasons, the exact “recipe” developed in the lab does not translate well to larger scale.
A list of key parameters and considerations to take into account when scaling up a bioprocess:
- Oxygen Transfer Rate (OTR) and kLa
- Heating/Cooling (Heat Transfer)
- Mixing (Impeller type, Speed, Shear)
- Pressure (Diastatic)
- Solubility of gases (Temperature, Partial Pressure)
- Foaming (Antifoam)
- Tank/Fermenter Aspect Ratio
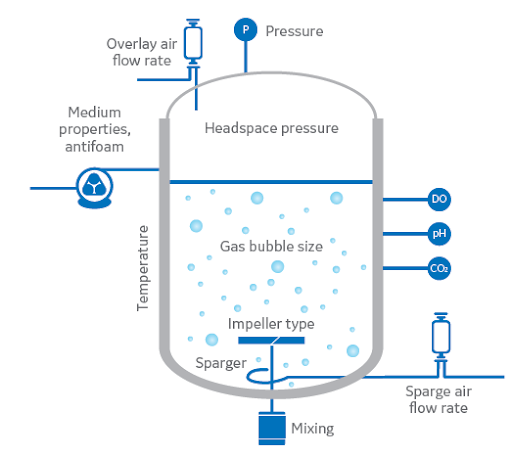
Other important considerations and challenges for scaling up a bioprocess:
- Sourcing and Optimizing Feedstocks/Media components (variability of raw materials)
- Feed rates and switching feed stocks
- Micro vs. Macro nutrients (purity and availability)
- Contamination/Sterility
- Fermentation By-products (inhibitory)
- Cleaning/CIP/SIP
- Utilities (appropriate for application, interruption of services)
- Equipment fouling/failure
- CMO/CRO – incompatibilities with process, equipment, and regulation
How Solaris Can Facilitate Scaling Up Your Bioprocess:
- Robust designs/equipment
- Custom engineered solutions
- Software
- Range of products/Scale
For large turnkey project, Solaris offers Clean In Place (CIP) and Steam In Place (SIP) skids integrated with your custom system.
Solaris also provides a highly adaptable pilot scale Tangential Flow Filtration unit known as Tytan, which is idea for downstream processing and integrated with your Solaris Biotech equipment.